Posted by Gregory J Block MSc PhD on Feb 3, 2015
Project summary and stated goals: The purpose of this study is to explore the possibility that FSHD biomarkers exist in human serum. We proposed to screen serum samples from FSHD-affected individuals and controls for proteins that varied in concentration and correlated with disease presence, and severity. I began this study by collecting serum samples from a cohort of 30 individuals (18 FSHD-affected and 12 unrelated, unaffected controls. Analysis of this cohort showed promising biomarker identification and provided the preliminary data to support a follow-up study (this proposal) to assay an additional 48 serum samples collected by Dr. Rabi Tawil at the University of Rochester. The vast majority of funding was to pay for screening using new technology coordinated by the company SomaLogic. Due to an unanticipated accident of one of Dr. Tawil’s lab members, acquisition of samples and initiation of screening was delayed significantly. Here we report the progress to date of the stated goals.
1. Determine if candidate biomarker levels correlate with an FSHD diagnosis. As a first step toward identifying biomarkers that correlate with disease severity we attempted to identify proteins whose level correlated with the presence of FSHD. Unfortunately, differences between our lab and Dr. Tawil’s lab in the approach to sample acquisition, preparation, and/or storage will prevent us from combining both cohorts. However, the samples collected in my lab, and the samples collected in Dr. Tawil’s lab were large enough to be analyzed separately and compared as independent studies from two sites. As in the earlier analysis presented as preliminary data for this proposal we did find several biomarkers that correlate with the presence of FSHD in a statistically significant manner. Of the 1129 proteins analyzed we found 164 that were differentially expressed between FSHD and control individuals. Narrowing this sample set to serum proteins that differed by 1.5 fold revealed a subset of 16 serum proteins that are significantly elevated in people affected by FSHD. When analyses of both cohorts were performed independently, several biomarkers overlapped between the data sets indicating that there are serum proteins that correlate with FSHD pathology. Serum biomarkers included Creatin Kinase (CK) and Carbonic Anhydrase (CA3), proteins previously shown to be elevated in FSHD-affected individuals. In addition novel biomarkers were discovered that may shed light on disease pathology and have a higher sensitivity and specificity for measuring FSHD severity and progression. We are continuing to revise the analysis of these data to determine how best to combine study results (Fig. 1).
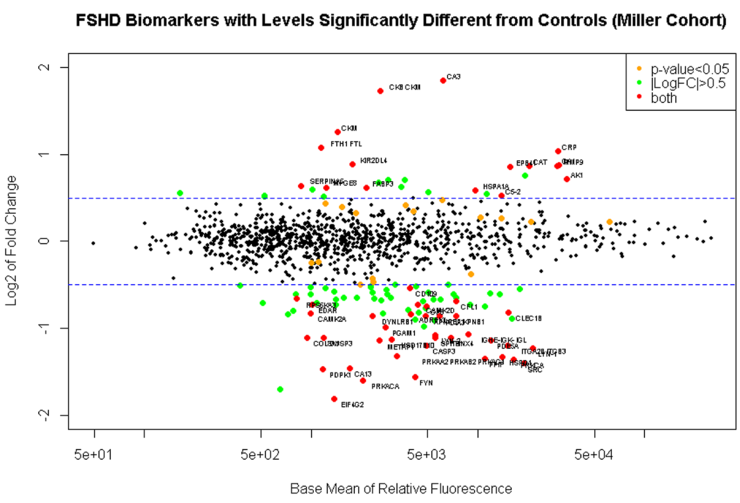
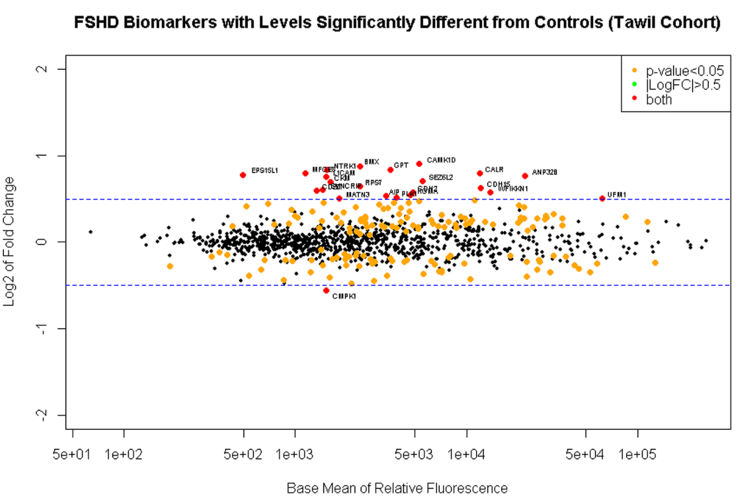
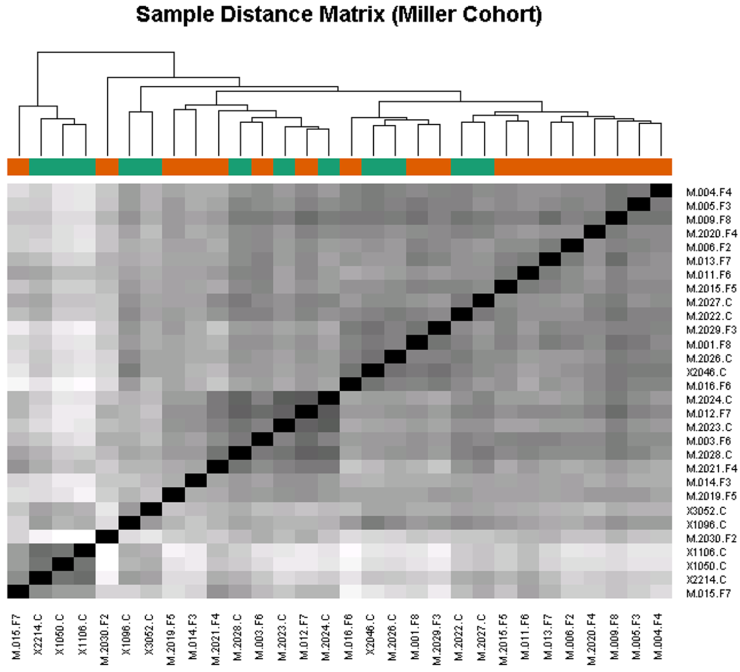
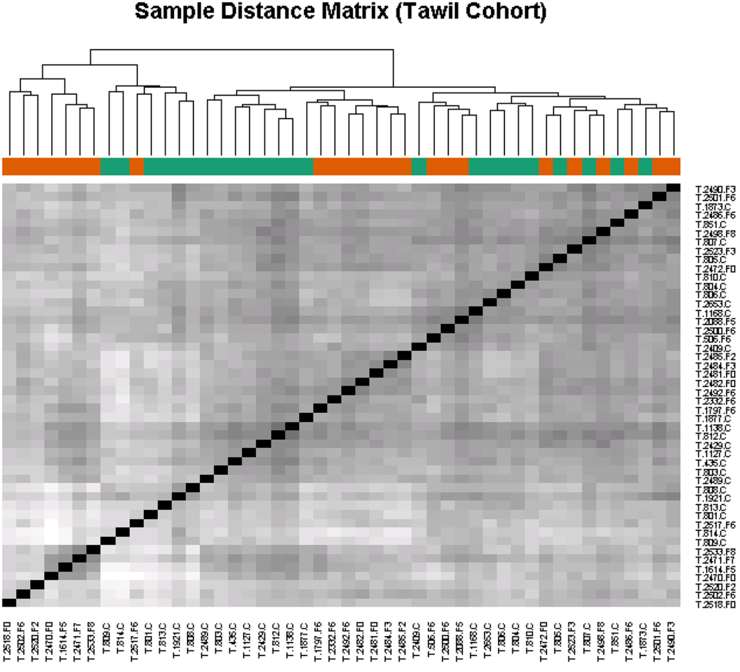
2. Determine if candidate biomarker levels correlate with disease severity. The high throughput approach used here will lend itself to the analysis of panels of biomarkers that may predict disease severity as a group, rather than the analysis of a single marker. Euclidean sample to sample distances calculated from the regularized log transformation of differences in relative fluorescence in the SomaLogic assay showed clustering of samples from FSHD-affected individuals having similar clinical severity scores (Fig. 2). This observation indicates that biomarker levels may also correlate with disease severity.
3. Determine if candidate biomarker levels correlate with disease progression. An ideal marker might change over the course of a year despite no noticeable difference in strength or symptoms in the person affected by FSHD. If we are able to identify biomarkers that meet our criteria, the next step will be to determine if biomarker levels change over time. Since significant time frames in the context of FSHD are measured in years, progress on this aim will be reported at a later date and if funds permit.
4. Determine if commercially available ELISA tests can detect and quantify the biomarker candidate for larger cohort studies. At the time of submitting this proposal SomaLogic did not provide customized restricted panels of somamers for follow-up and confirmatory studies. However, during a recent conference call with the company to discuss our data, I was told that customized panels were being requested by a number of researchers and that the possibility of developing an FSHD-specific panel will likely occur in the next year. Antibodies used for Elisa tests may not always be a good follow-up for somamer analysis since the accessible binding sites in the context of serum proteins may differ between somamers and antibodies. Thus we will attempt to perform future follow-ups with specialized somamer panels as they become available.
Summary and Future Direction: Overall we’ve made progress that is essentially on target with the proposed time line shown in our original proposal. I think further analysis of these samples by grouping them according to clinical severity score may be a more sensitive way of identifying key biomarkers that reflect the ongoing pathology of FSHD. It makes sense that an individual with FSHD with a clinical severity score of 0 will have serum biomarker concentrations more similar to controls than someone with moderate or severe FSHD. Once we’ve arrived on a set of markers that appears to be informative we plan to solicit the construction of an FSHD panel from SomaLogic for use in a larger cohort. We also will continue to follow the individuals from the Miller Cohort as they return for repeat MRI imaging and at some point we can correlate biomarker levels with disease pathology quantified by MRI. Thank you for your support.
See Grant Identification of Serum Biomarkers for Measurement of FSHD Progression





Connect with us on social media