Posted by Amanda Rickard on Dec 20, 2016
Reprinted with permission
Contact: Mark Johnson
Sr. Director, Investor Relations
mjohnson@atyrpharma.com
+1-858-223-1163
aTyr Pharma Reports Promising Signals of Clinical Activity in Multiple Rare Genetically Distinct Myopathies with Resolaris™ in Exploratory Trials
- Improved Muscle Strength Observed in 50% to 78% of Patients in Two Rare Genetically Distinct Myopathies -
- Resolaris Continues to Demonstrate a Generally Well Tolerated Safety Profile in All Trials -
- Conference Call and Webcast Featuring Guest Speaker Professor John Vissing, MD, at 8:30 a.m. (EST) Today -
SAN DIEGO – December 13, 2016 – aTyr Pharma, Inc. (Nasdaq: LIFE), a biotherapeutics company engaged in the discovery and development of Physiocrine-based therapeutics to address severe, rare diseases, today announced clinical results from exploratory trials assessing the safety and potential activity of Resolaris™, including:
- Top-line results from a completed Phase 1b/2 trial for adult patients with limb-girdle muscular dystrophy 2B (LGMD2B/dysferlinopathy) or facioscapulohumeral muscular dystrophy (FSHD) (the “004 Trial”);
- Interim data from an ongoing Phase 1b/2 trial with early onset FSHD (the “003 Trial”); and
- Interim data from an ongoing long-term safety extension study (the “005 Trial”) for patients from aTyr’s adult FSHD trial completed earlier this year (the “002 Trial”).
The results announced today highlight the potential of Resolaris, an immuno-modulator of activated T cells, as a single treatment for multiple rare myopathies with an immune component (RMIC).
"Congratulations to our team, collaborators and patients that helped us accomplish the fundamental objectives for these clinical trials: (1) to demonstrate the safety and tolerability of our product candidate, Resolaris, across different RMICs and (2) to explore different readouts of potential clinical activity and product candidate activity in different RMICs,” said John Mendlein, PhD, CEO of aTyr Pharma. “We are very pleased to see RMIC patients with two entirely different genetic etiologies showing improvement in muscle strength in 3 months as measured by manual muscle testing. Taken together, our clinical data supports the exciting potential of Resolaris as a single treatment for multiple rare myopathies with an immune component and we believe our clinical results will help inform and direct the future clinical development of Resolaris in RMICs. Finally, we wish to personally thank all the FSHD and LGMD patients and health care professionals who have participated in our trials."
"aTyr’s data, which shows potential clinical activity in genetically distinct myopathies characterized by an immune component, warrants additional clinical investigation,” said Dr. John Vissing, Professor of the Department of Neurology at the University of Copenhagen and an investigator in aTyr’s 004 Trial. “It is supportive of the hypothesis that administering a naturally occurring muscle homeostasis protein with immuno-modulator activities, which is normally secreted by skeletal muscle cells, has the potential to help patients across many rare myopathies with divergent genetic etiologies of disease."
Clinical Activity Assessments: As part of clinical assessments in these studies, manual muscle testing (MMT), a validated assessment tool that measures muscle strength, was performed across 14 selected muscle groups. In addition, a validated patient reported outcome measure designed specifically for neuromuscular disease, the individualized neuromuscular quality of life (INQoL) questionnaire, was utilized. Note that an increase in MMT score represents an increase in muscle strength, whereas a decrease in INQoL score represents a decrease in disease burden. Given that the 003, 004 and 005 Trials are small and open-label in nature and that the clinical assessments expressed in this release, MMT and INQoL, although clinically validated, are subject to variability over time, including intra-patient and inter-physician variability, it is important to temper any definitive conclusions made with respect to the clinical activity of Resolaris.
LGMD2B/FSHD (004) Trial: This international Phase 1b/2 clinical trial at 6 clinical sites was an open-label, intra-patient, placebo run-in, dose escalation study designed to assess the safety, tolerability, immunogenicity and exploratory assessments of clinical activity of intra-patient dose escalations to twice weekly (biw) intravenous infusions of Resolaris in LGMD2B and FSHD adult patients. Patients were assigned to two treatment groups each with 12 weeks of treatment:
- Group A: 4 patients with FSHD received infusions of Resolaris with the highest dosing up to 1.0 mg/kg biw for a period of 4 weeks; and
- Group B: 10 patients with LGMD and 4 patients with FSHD received infusions of Resolaris with the highest dosing up to 3.0 mg/kg biw for a period of 4 weeks.
Manual Muscle Testing, MMT, Assessment 004 Trial:
(See Figure 1 and Figure 2)
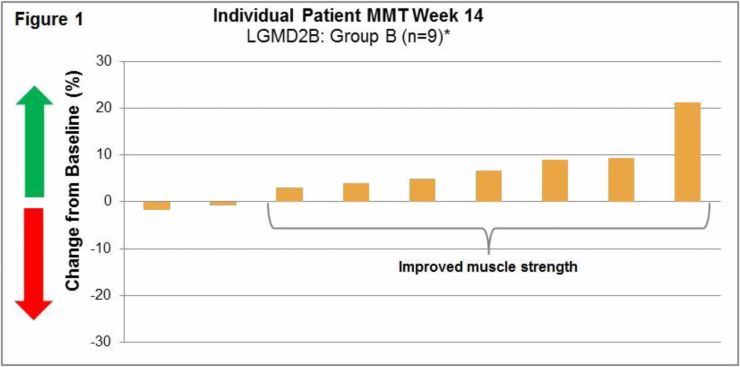
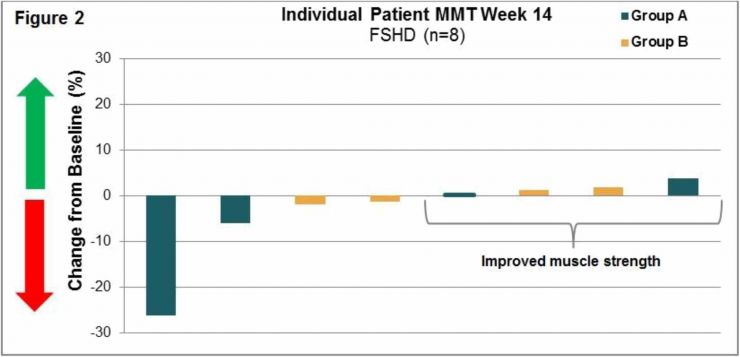
Individualized Quality of Life, INQoL, Assessment 004 Trial:
- LGMD2B Patients: Overall INQoL score was relatively stable for these 10 patients with overall approximately equal proportions of patients with decreases in disease burden compared to increases in disease burden
- FSHD Patients: Overall INQoL score was relatively stable for these 8 patients with 5 of 8 patients presenting with a small decrease in disease burden over the length of the trial
Biomarker Summary in 004 Trial:
Various exploratory biomarkers (including targeted muscle T2 and STIR MRI and various plasma proteins) did not generally establish sufficiently high or consistent levels or robust signals across a sufficient number of patients to determine test article effects. Peripheral cell based biomarkers will be assessed at a later date. Targeted muscle T2/STIR MRI will not be prioritized as a biomarker in the near-term.
Early Onset FSHD (003) Trial: This ongoing international, multi-center, open-label, intra-patient, placebo run-in, dose escalation Phase 1b/2 study is designed to evaluate the safety, tolerability, immunogenicity and exploratory assessments of clinical activity of Resolaris at weekly doses of 0.3, 1.0 and 3.0 mg/kg in patients with early onset FSHD for a total of 12 weeks.
An interim data cut was conducted for the first four early-onset FSHD patients that completed treatment with Resolaris (age range of 16 to 20).
Manual Muscle Testing, MMT, Assessment 003 Trial:
(See Figure 3)
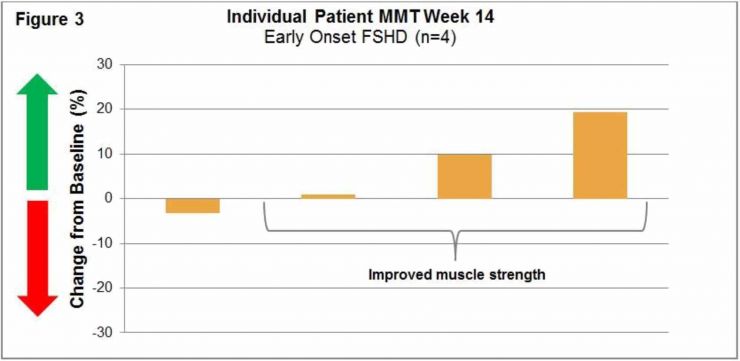
Individualized Quality of Life, INQoL, Assessment 003 Trial: Patient’s INQoL scores were relatively stable. Two patients had slight decreases in disease burden and one patient showed an increase. The fourth patient did not have a baseline INQoL assessment.
Adult FSHD Long Term Safety Extension (005) Trial: This ongoing international, multi-center, open-label extension clinical trial is designed to assess the long-term safety, effects on biomarkers and systemic exposure of Resolaris in adult FSHD patients from the completed 002 Trial. Patients receive weekly doses of 3.0 mg/kg on an ongoing basis.
- 3 of the 9 patients enrolled from the adult FSHD (002) Trial are still receiving treatment
- Of the 4 patients who received at least 6 months of therapy in the 005 Trial, there were no significant trends in worsening or improvement in either MMT or INQoL scores
- Peripheral cell based biomarkers and other biomarkers will be assessed at a later date
Safety and Tolerability Summary
- As of December 1, 2016, 44 patients have received Resolaris, across all trials, for a total drug exposure of 149 patient months
- Resolaris continues to demonstrate a favorable safety profile and was generally well-tolerated across all doses tested in adult FSHD, early onset FSHD (younger population ages 16 – 25) and adult LGMD2B for 3 months of dosing, as well as with long-term exposure in adult FSHD patients
- No Serious Adverse Events (SAEs) were reported by investigators in the 003, 004 and 005 Trials
- Adverse Events (AE) reported were in general mild or moderate in intensity
- No notable differences in AEs between adult FSHD, adult LGMD2B and early onset FSHD patients
- Protocol related discontinuations
- Per protocol, patients:
- are not medicated before or during infusion for infusion related reactions (IRRs);
- discontinue upon occurrence of an IRR and 4 FSHD patients and 1 LGMD patient discontinued for this reason; and
- reaching Jo-1 antibody unit levels above the designated protocol cut-off must discontinue treatment and 5 FSHD patients discontinued for this reason.
- 1 LGMD patient discontinued from the 004 Trial for non-drug related reasons
- All IRRs were mild to moderate and transient
- Elevated Jo-1 antibody observations were without associated clinical symptoms
- After changing the infusion protocol to a 90-minute infusion, 9.1% experienced IRRs (previously the rate was 16.7% with an infusion rate of 30 minutes)
- The overall discontinuation rate in the 003, 004 and 005 Trials under all protocols is 11 out of 35 patients (31%)
- In the 003, 004 and 005 Trials, low level anti-drug antibody (ADA) titer signals were observed in 19 of 35 (54%) patients dosed (13 FSHD and 6 LGMD); these low level signals did not warrant neutralizing ADA assays and no clinically significant findings were associated with these ADA assay signals
- Per protocol, patients:
Resolaris Summary
After reviewing the entire clinical program for Resolaris, which spans 44 patients in four separate interventional trials, aTyr believes that these results are supportive of the advancement of Resolaris as a single treatment for various rare myopathies with an immune component. Next steps for the company include completing the evaluation of the 003, 004 and 005 biomarker data, particularly peripheral cell based data using one or more mechanistic assays currently under development at aTyr for agonists of the Resokine pathway and T cell activity. Future trials will be designed using one or more of these mechanistic assays, as well as the option to assess local immune components in skeletal muscle directly with biopsies. In addition, aTyr plans to meet with the FDA in 2017 to discuss a regulatory path towards a Biologics License Application (BLA).
2017 Outlook
In 2017, aTyr looks forward to:
- Emphasizing one RMIC indication in the Resolaris program to enhance its multiple rare myopathies, single treatment strategy;
- Advancing its iMod.Fc program into the clinic (including completion of GLP safety studies and GMP manufacturing for early clinical work) for rare lung diseases; and
- Furthering its clinical and R∓D pipeline by partnering one or more of its programs, thereby driving value for its stockholders and ultimately making meaningful medicines available for patients.
Conference Call and Webcast Information
Today at 8:30 a.m. EST, aTyr Pharma will host a conference call and webcast with an accompanying slide presentation to discuss the results of the Phase 1b/2 program of Resolaris™. The live webcast and slide presentation will be available on the “Investors” section of the company website at www.atyrpharma.com. Joining aTyr Pharma management will be Professor John Vissing, M.D., Professor of Neurology at the University of Copenhagen, Denmark. To access the call, please dial (877) 870-4263 (domestic) or (412) 317-0790 (international) and ask to join the aTyr Pharma call. A replay of the webcast will be archived on the company's website following the call.
About Resolaris™
aTyr Pharma is developing Resolaris as a potential first-in-class intravenous protein therapeutic for the treatment of rare myopathies with an immune component. Resolaris is derived from a naturally occurring protein released in vitro by human skeletal muscle cells. aTyr believes Resolaris has the potential to provide therapeutic benefit to patients with rare myopathies with an immune component characterized by excessive immune cell involvement.
About iMod.Fc
aTyr Pharma established a discovery program to leverage its knowledge of the Resokine pathway to vary exposure and activity of the iMod domain through protein engineering. aTyr’s Fc fusion experiments helped delineate how to enhance the exposure of the iMod domain of Resokine while maintaining activity and provide insights into this domain harboring immuno-modulatory activity. aTyr plans to test the potential of this molecule in lung disease by developing it as a potential therapeutic for patients with rare pulmonary diseases with an immune component (RPICs).
About LGMD2B / Dysferlinopathy
Limb girdle muscular dystrophy (LGMD) refers to a group of rare genetic myopathies, of which there are more than 20 different subtypes, none with approved therapies. LGMD affects an estimated 16,000 patients in the U.S., approximately 3,000 of who have LGMD2B. LGMD2B is a recessive genetic disease caused by a toxic loss of function in the dysferlin gene. Patients experience progressive debilitating muscle weakness and atrophy as well as immune cell invasion in the skeletal muscle.
About FSHD
Facioscapulohumeral muscular dystrophy (FSHD) is a rare genetic myopathy affecting an estimated 19,000 people in the United States for which there are no approved treatments. It is caused by a toxic gain of function in the DUX4 gene. The primary clinical phenotype of FSHD is debilitating skeletal muscle deterioration and weakness. The symptoms of FSHD often appear early in the face, shoulder blades, upper arms, lower legs and trunk, and can affect certain muscles while adjacent muscles remain healthy. In addition to muscle weakness, FSHD patients often experience debilitating fatigue and chronic pain. The disease is typically diagnosed by the presence of a characteristic pattern of muscle weakness and other clinical symptoms, as well as through genetic testing.
About Early Onset FSHD
While FSHD can manifest at any age, the onset of symptoms in many patients occurs before the age of 18. We refer to this patient population as early onset FSHD. aTyr has selected those patients with onset of symptoms before the age of ten for its current clinical trial. Within the early onset population are individuals with symptom onset at less than five years of age, with progression in disease prior to age ten. These individuals have generally the most severe muscle symptoms and extra-muscular manifestations such as auditory deficits and retinal complications that may result in vision loss. This sub-group of early onset patients are often referred to as having “infantile onset” FSHD. Estimates of prevalence vary; however, aTyr believes the “infantile onset” FSHD population is approximately 1,000 in the U.S.
About aTyr Pharma
aTyr Pharma is engaged in the discovery and clinical development of innovative medicines for patients suffering from severe, rare diseases using its knowledge of Physiocrine biology, a newly discovered set of physiological modulators. The Company's lead candidate, Resolaris™, is a potential first-in-class intravenous protein therapeutic for the treatment of rare myopathies with an immune component. aTyr has built an intellectual property estate, to protect its pipeline, comprising over 80 issued or allowed patents and over 230 pending patent applications that are owned or exclusively licensed by aTyr, including over 300 potential Physiocrine-based protein compositions. aTyr's key programs are currently focused on severe, rare diseases characterized by immune dysregulation for which there are currently limited or no treatment options. For more information, please visit http://www.atyrpharma.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Litigation Reform Act. Forward-looking statements are usually identified by the use of words such as "anticipates," "believes," "estimates," "expects," "intends," "may," "plans," "projects," "seeks," "should," "will," and variations of such words or similar expressions. We intend these forward-looking statements to be covered by such safe harbor provisions for forward-looking statements and are making this statement for purposes of complying with those safe harbor provisions. These forward-looking statements, including statements regarding the potential of Resolaris™ or iMod.Fc, the ability of the Company to undertake certain development activities (such as clinical trial enrollment and the conduct of clinical trials) and accomplish certain development goals, the timing of initiation of additional clinical trials and of reporting results from our clinical trials and reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a variety of risks and factors that are beyond our control including, without limitation, risks associated with the discovery, development and regulation of our Physiocrine-based product candidates, as well as those set forth in our most recent Annual Report on Form 10-K for the year ended December 31, 2015 and in our subsequent SEC filings including our most recent Quarterly Report for the quarter ended September 30, 2016. Except as required by law, we assume no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise.





Connect with us on social media