Posted by Friends of FSH Research on May 15, 2019
Through your generosity, and with matching funds, we raised $21,500 to help fund Evaluators for the FSHD Clinical Trial Research Network (CTRN).
See grant: CTRN Evaluator Training
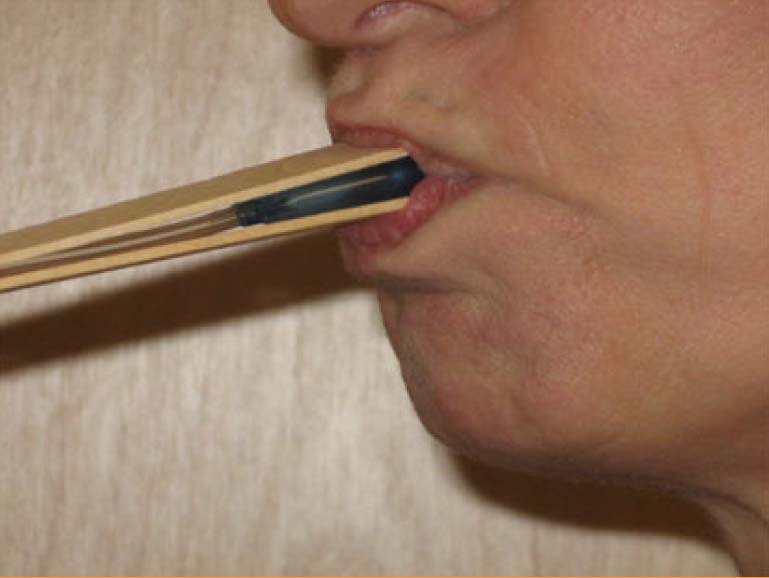
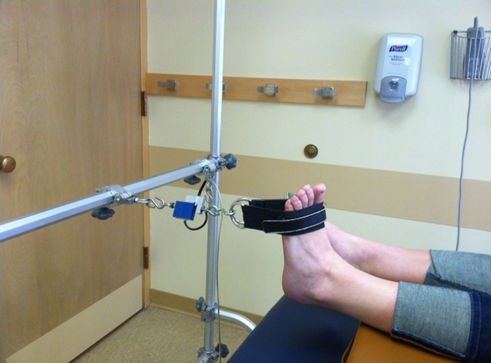
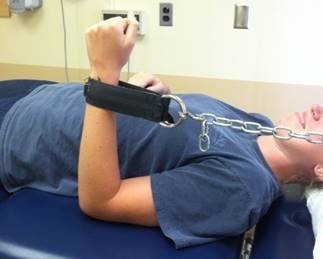
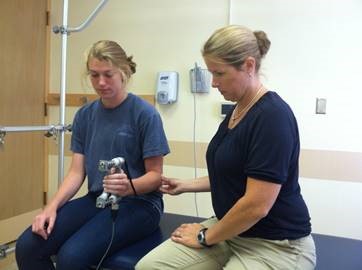
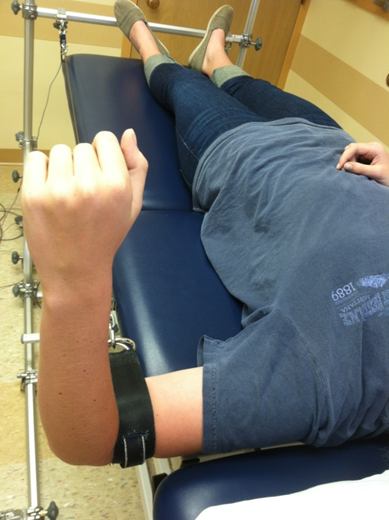
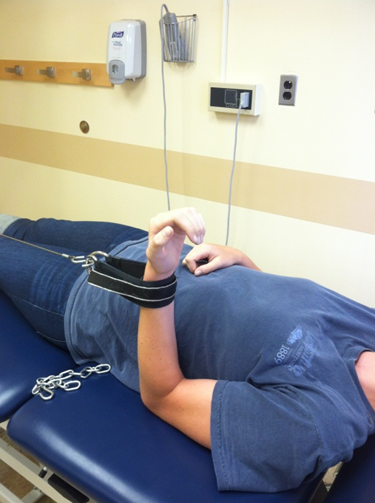
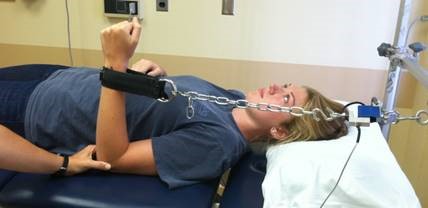
Evaluators are physical therapists (PT) that are a key component in our clinical trial research network as they perform clinical outcome assessments needed in research studies. Clinical evaluators have a background in physical therapy and perform functional assessments such as: 6 minute walk, 4 stair climb, timed up and go; strength testing: manual muscle testing, quantitative muscle testing; respiratory testing and facial strength testing.
It is essential clinical evaluators perform study assessments in a standardized manner, across all sites, to maximize reliability and minimize variability of data collected for research studies.
Cost of Training Each Evaluator
The new clinical evaluator receives one on one training and practice with the lead FSHD CTRN evaluator at the University of Rochester Medical Center.
- Air travel $700 - Requires travel to and from URMC for training with lead evaluator.
- Hotel $250 - Requires at least one overnight stay at URMC for specialized training. Training is individualized for each evaluator.
- Lead Evaluator $300
- Clinical Research Center time $300
- Honorarium for volunteers for live testing: $150 - In order to properly train new evaluators, training on live volunteers is needed to conduct many of the assessments required for training.
- Total $1,700
FSHD Clinical Trial Research Network
The FSHD CTRN is a consortium of academic centers (8 in the United States and 3 in Europe) with expertise in FSHD clinical research or in conducting neuromuscular clinical trials. These sites have a well established clinical trial infrastructure including a principal investigator, clinical research coordinator, clinical evaluator or physical therapist (PT) and institutional research resources.
Training Evaluators covers one small, but crucial piece of the puzzle on the way to clinical trials. In addition, expanding the FSHD CTRN into regions not currently covered will allow greater access to people with FSHD and the ability to increase the network of clinical trial ready sites across the world. As we move closer to therapeutic research trials, it is essential to have a large network of clinical trial ready sites and greater access to people with FSHD.





Connect with us on social media