Investigator: Barbara D Page PhD
Category: Research - Basic
During my past year as an FSH Research Scholar in Dr. Stephen Tapscott’s laboratory at the Fred Hutchinson Cancer Research Center, I have focused on using two different model organisms to better understand facioscapulohumeral muscular dystrophy (FSHD). The goals of my research are:
- to develop new therapies to treat patients with this disease by performing high throughput small molecule screens and
- to provide greater understanding as to how DUX4, a gene that is inappropriately expressed in patients with FSHD, functions by studying its role in normal development.
Recent experiments suggest that FSHD may be caused in part by the inappropriate expression of DUX4 due to a loss of gene silencing. Therefore, a possible line of therapy for patients with FSHD would be the use of drugs that could reestablish silencing of the DUX4 gene. As a first step to identify such drugs, I am using the yeast S. pombe to screen for small molecules that can increase gene silencing. During this last year, I have designed and successfully optimized this screen to work in a high throughput format. During the next year, working in collaboration with researchers at the National Institutes of Health’s Chemical Genomics Center, we will initiate a pilot screen for small molecules that can enhance gene silencing. These studies will lay the foundation for testing a much larger library of thousands of compounds to identify potential therapeutics for patients with FSHD.
My second project involving the model organism C. elegans takes advantage of the numerous genetic tools available in this organism to investigate how genes like DUX4 function in normal development. We have discovered a DUX4-like gene in C. elegans, which we have named dux-1. My studies have shown that the C. elegans dux-1 gene is critical for male fertility. In the male germ line, the dux-1 gene and genes that regulated cell death work together to remove parts of the spermatocyte in order to form compact, functional sperm. Additionally, I have observed that inappropriate expression of dux-1 during development of the embryo causes muscle paralysis. Taken together, these results suggest that dux-1 could lead a program of organized cellular destruction, and that muscle is particularly sensitive to the activation of this pathway. Our above observations of dux-1 function in C. elegans suggest that DUX4 in human cells might orchestrate a “demolition squad” to remove and recycle cellular components. Greater detail about dux-1’s targets and collaborators as well as understanding the sensitivity of muscle to its expression will hopefully lead to new lines of promising experimental research for patients affected with FSHD.
Specific Aims
Aim 1 will test the hypothesis that a small molecule can be used to increase epigenetic silencing. We had previously obtained and characterized an S. pombe yeast strain with marker genes inserted flanking the mating type locus on chromosome II. This strain had the ura4 and ade6 genes located at the boundaries of the mating type repressive chromatin. We had also confirmed that mutations in the ribonucleotide reductase pathway (cdc22 and tds1) allowed for the spreading of repressive heterochromatin, as demonstrated by the 5-FOA resistance of this strain. After performing a number of optimization steps (cell number, media composition, growth conditions, ATP luciferase assay development), we successfully established a liquid media-based assay in a 96-well plate format. Using these testing conditions, we identified a compound that could serve as a positive in our screen (hydroxyurea). Given these promising preliminary results, we began a collaboration with Dr. Marc Ferrer, PhD at the NIH Chemical Genomics Center (NIHCGC) to initiate the beginning of a high throughput small molecule screen for compounds capable of increasing repressive chromatin structures. Since this assay at the NIHCGC required use of a 1536-well format, Dr. Ferrer and his colleagues began to optimize the miniaturization protocol for this platform. Initial attempts at this process demonstrated a high degree of well-to-well variability that would require further optimization. While this was occurring, the NIHCGC underwent a change with regard to requirements for funding collaborative screens. We had previously planned to write an internal NIH RO3 grant with his group to perform the high throughput screen, but unfortunately this funding mechanism was terminated. Given that the yeast screen still required significant optimization and the change in funding mechanism, this Aim was suspended to concentrate on Aim 2 for the remainder of the award period.
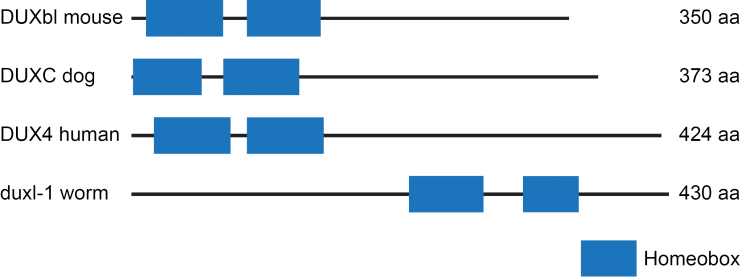
Aim 2 will test the hypothesis that a DUX4 ortholog from the model organism C. elegans can be used to understand the pathogenesis of FSHD. The C. elegans gene duxl-1 (DUX [vertebrate dual homeobox] like) encodes a dual homeobox protein with similarity to the human protein DUX4. We decided to take advantage of this similarity and the excellent genetic, molecular, and developmental biology of C. elegans to investigate the function of a dual homeobox protein. Because C. elegans has been extraordinarily useful in dissecting the molecular components and their functions in such biological processes as germ line stem cell regulation, programmed cell death, neuronal path finding and RNA interference, the C. elegans research community has organized collections of mutants and gene fusions that are available to all researchers. We obtained useful reagents such as a deletion of the duxl-1 gene and a gene fusion between the duxl-1 promoter and gfp. The strain containing duxl-1(tm956), a deletion allele of duxl-1, was studied under different conditions to determine when the DUXL-1 protein functions. The duxl-1 promoter fusion to gfp was examined to determine which tissues express DUXL-1. Using these tools, we have found that duxl-1 affects fertility and anatomy of the gonad. Our results suggest that duxl-1 plays a regulatory role in cellular remodeling, a process that requires the careful removal of part of a cell without damaging the rest of the cell. If the function of duxl-1 in C. elegans has similarity with the function of DUX4 in human cells, then the ectopic expression of DUX4 protein in skeletal muscle, as observed in patients with FSHD, might inappropriately initiate cellular remodeling of otherwise healthy, normal muscle. Immune cells, such as macrophages, could recognize this signal, and this response could cause slow loss of muscle tissue. It is hoped that this insight will lead to new therapies to prevent muscle destruction in patients with FSHD.
Layman Description
As a Friends of FSH Research Scholar, I have focused on using two different model organisms to identify new therapies for facioscapulohumeral muscular dystrophy (FSHD) and better understand how this disease occurs. Recent experiments suggest that FSHD may be caused in part by the inappropriate expression of the gene DUX4 due to a loss of gene silencing. Therefore, a possible line of therapy for patients with FSHD would be the use of drugs that could reestablish silencing of the DUX4 gene. To identify such drugs, my first project focused on developing an assay using the model organism S. pombe (a single-celled yeast) as a tool to screen for small molecules that can increase gene silencing. Our pilot studies suggest that this assay can be used for the large scale testing of compounds to identify potential therapeutics for patients with FSHD. My second project took advantage of the numerous genetic tools available in the model organism C. elegans (a very small worm) to investigate how genes like DUX4 function in normal development. We discovered a DUX4-like gene in C. elegans, which we named duxl-1. Our results suggest that duxl-1 functions in the remodeling of tissues by regulating an "eat me" signal important for organized cellular destruction. If DUX4 in human cells acts similarly, then the inappropriate expression of DUX4 in skeletal muscle, as observed in patients with FSHD, might mislabel healthy muscle cells with the "eat me" signal. Immune cells in the body would recognize this signal, leading to the slow loss of muscle tissue. It is hoped that this insight will lead to new therapies to prevent muscle destruction in patients with FSHD.
—Barbara D Page
See Progress Report






Connect with us on social media